Introduction
The poultry industry, a significant global producer of chicken and eggs (with production and consumption elevating significantly over the past two decades), faces mounting concerns from researchers and consumers alike regarding antibiotic resistance. The widespread historical use of antibiotics as growth promoters in food animals since the 1940s and 1950s has unfortunately fostered the emergence of antibiotic-resistant pathogens, posing risks to both animal and human health, including gut microbiota disruption, hypersensitivity reactions, and potential carcinogenicity. This critical challenge necessitates the development of safe and effective alternatives to conventional antibiotic growth promoters (AGPs).
One promising avenue lies in the valorization of cheese whey, a dairy by-product generated in immense quantities (180–200 million metric tons globally in 2023).
This research introduces Fermented Whey Peptides (FWP), derived from lactic fermented cheddar cheese whey using Limosilactobacillus fermentum (M4, GenBank Accession Number: MF951096). FWP leverages the nutritional richness of whey while offering a pre-digested, lactose-free, and bioactive-peptide-rich feed additive, readily available year-round. Fermentation, conducted at 37°C for 48 hours, hydrolyzes whey proteins into peptide fragments (3 kDa to 10 kDa), enhancing bioavailability and releasing bioactive compounds with demonstrated antioxidative, antidiabetic, and anti-inflammatory properties. This study, involving 96 one-day-old mixed-sex commercial broiler chicks in a Completely Randomized Design, investigated FWP as a nutraceutical supplement to basal diets, analyzing its effects on growth performance, blood parameters, relative organs (via histological analysis), and metagenomic profiles of broiler cecal contents, aiming to establish it as a viable, biohazard-free substitute for antibiotics in organic poultry production.
Material and Method
This study investigated the effects of fermented whey peptides (FWP) on broiler chickens. Cheese whey, sourced from Vidya Dairy, Anand, India, was sterilized at 121°C (250°F) under 15 psi for 15 minutes, then cooled and stored at 5±1°C. A pure culture of Limosilactobacillus fermentum (M4, MF951096) was inoculated into the sterilized whey at 2.5% and fermented at 37°C for 48 hours. FWP was extracted via centrifugation at 10,000 rpm for 30 minutes at 4°C. Supernatants were ultrafiltered using Amicon® Milipore membranes with 10 kDa and 3 kDa cut-offs at 5000 rpm for 30 minutes at 4°C, yielding >10 kDa, <10 kDa, and <3 kDa peptide fractions. Samples were stored at −20°C.
The in vivo study, adhering to CPCSEA and ARRIVE guidelines (Approval no. IAEC385/PRS/2022), involved 96 one-day-old mixed-sex commercial broiler chicks (initial BW 56.07±3.21 g) from Venky’s India. A Completely Randomized Design (CRD) was employed, with four treatments, each having four replicates of six birds (6×4×4). Treatments were: T1 (Control: basal diet + commercial probiotics + immunomodulator for 42 days); T2 (basal diet + <3 kDa FWP for 7 days); T3 (basal diet + <10 kDa FWP for 7 days); T4 (basal diet + >10 kDa FWP for 7 days). Freshly prepared FWP (1 mL) was orally administered daily from day 8 to day 15. Basal diets, formulated from corn and soybean mash per Hati et al. (2020) and Table S1, met broiler starter, grower, and finisher nutritional requirements and were provided ad libitum.
Growth performance (BW, FI, BWG, FCR) was assessed weekly from 0 to 42 days. Blood samples were collected from wing veins on day 42 (4 birds/replicate). Hematological parameters (RBC, WBC, Hb, HCT, MCV, MCH, MCHC) were analyzed using a Mindray BC 2800 Vet hematology analyzer. Serum biochemical parameters (glucose, cholesterol, LDL, HDL, VLDL, TG, TC) were determined using commercial kits and an auto analyzer. Histological analysis involved preparing 5 mm x 10 mm x 20 mm sections of intestine, liver, and heart, fixed in 10% neutral buffered formalin (NBF), processed, embedded in paraffin, sectioned (5–6 μm) using a Microtome, and stained with Hematoxylin and Eosin (H & E).
For metagenomic analysis, cecal contents were collected post-euthanasia via cervical dislocation, mixed 1:1 with RNA protect Bacterial reagent (Qiagen), and stored at −20°C then −80°C. Metagenomic DNA was extracted using QIAamp Fast DNA stool kit (Qiagen), quantified by Qubit 3.0 fluorometer, and quality checked by agarose gel electrophoresis. 16S amplicon sequencing targeting the V3-V4 region was performed using 341F and 785R primers, following Illumina 16S Metagenomics library preparation guidelines. Sequencing data was analyzed in R v4.2.2 using DADA2 package v1.26.0 for ASV generation and taxonomic assignment, followed by phyloseq R package v1.46.0 for microbiome analysis, with ASVs having less than 6 reads or present in only one sample filtered out. Statistical analysis for hematological and biochemical data involved one-way ANOVA followed by Duncan’s test, with results expressed as mean ± SEM at p<0.05.
Results
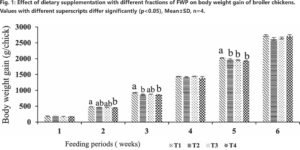
Effect of fermented cheese whey fractions supplementation on growth performance of broilers
No significant (p < 0.05) effect was observed on body weight gain at the 6th week across all groups (Control T1, FWP-fed T2, T3, T4). Numerically, the control group (T1: 2730.13±28.84 g) exhibited a higher average body weight than FWP-fed groups (T3: 2651.63±32.98 g; T4: 2707.03±51.34 g; T2: 2626.38±40.74 g) as shown in Fig. 1. However, FWP fractions did show a significant (p < 0.05) impact on body weight gain during the 2nd, 3rd, and 5th weeks of the study. Specifically, at the 4th week, the group supplemented with <10 kDa FWP (T4) recorded the highest body weight gain of 1441.42±7.87 g, indicating that the T4 diet group promoted faster muscle growth and improved gut development. Feed consumption peaked across all dietary groups between the 3rd and 6th weeks, consistent with the chicks’ increased energy needs during development.
 Highest feed consumption for all dietary groups occurred between the 3rd and 6th weeks as shown in Fig. 2, coinciding with the chicks’ increased energy demands for growth. The control group (T1) exhibited the highest feed consumption, while the group fed with >10 kDa FWP (T4) consumed the least. This difference in intake is likely due to changes in palatability; the fermentation of cheese whey with L. fermentum (M4) reduced undesirable lactose, producing lactic acid and aromatic compounds, thus enhancing the flavor and carbohydrate solubility of the FWP.
Highest feed consumption for all dietary groups occurred between the 3rd and 6th weeks as shown in Fig. 2, coinciding with the chicks’ increased energy demands for growth. The control group (T1) exhibited the highest feed consumption, while the group fed with >10 kDa FWP (T4) consumed the least. This difference in intake is likely due to changes in palatability; the fermentation of cheese whey with L. fermentum (M4) reduced undesirable lactose, producing lactic acid and aromatic compounds, thus enhancing the flavor and carbohydrate solubility of the FWP.
Overall, FWP-containing diets led to elevated body weight gain despite reduced feed intake compared to the control. This improved efficiency is attributed to FWP’s superior nutritional profile, offering high-quality protein, essential amino acids, and branched-chain amino acids (BCAAs), known for their role in muscle protein metabolism and overall growth. Bovine whey protein’s balanced essential amino acid composition, enhanced digestibility, and high biological value contribute to this. Beyond providing amino acids, FWP’s bioactive peptides (2–20 amino acid residues) exert antimicrobial, antioxidant, antihypertensive, and immunomodulatory effects, promoting intestinal health and disease resistance, thereby enhancing feed efficiency. The T4 group, specifically, showed enhanced gut development and accelerated muscle growth, resulting in the highest observed body weight gain. All FWP diet groups demonstrated increased feed consumption and body weight gain until the 6th week, with FWP diets proving more palatable than the standard diet. Importantly, the addition of whey protein fractions did not affect the mortality rate in any FWP treatment groups (T2, T3, T4). The >10 kDa FWP fraction (T4) particularly improved overall body weight gain by 42 days of age.
 Regarding Feed Conversion Ratio (FCR), a key indicator of feed quality and weight gain efficiency, the control group (T1) exhibited a significantly higher average FCR at the 4th week compared to other treatment groups (p < 0.05) as shown in Fig. 3. While no significant difference in FCR was observed over the entire study period, the T1 group maintained the highest FCR (1.95) at the 6th week, numerically higher than T2 (1.78), T4 (1.67), and T3 (1.67). This higher FCR in the control group was linked to its higher feed consumption. The lower FCR observed in FWP-fed groups is potentially due to the satiety-inducing effects of whey-derived peptides, leading to reduced overall feed intake.
Regarding Feed Conversion Ratio (FCR), a key indicator of feed quality and weight gain efficiency, the control group (T1) exhibited a significantly higher average FCR at the 4th week compared to other treatment groups (p < 0.05) as shown in Fig. 3. While no significant difference in FCR was observed over the entire study period, the T1 group maintained the highest FCR (1.95) at the 6th week, numerically higher than T2 (1.78), T4 (1.67), and T3 (1.67). This higher FCR in the control group was linked to its higher feed consumption. The lower FCR observed in FWP-fed groups is potentially due to the satiety-inducing effects of whey-derived peptides, leading to reduced overall feed intake.
FWPs offer multiple functional benefits beyond those of typical prebiotics and probiotics, which primarily support gut immunity. FWP contains essential amino acids, organic acids, minerals, vitamins, and bioactive whey peptides with antioxidant and antidiabetic properties. This multi-faceted functionality contributes to enhanced immunity, improved gut microflora, and a better blood profile in the long term.
Similar studies support these findings: Hilmi et al. (2018) reported that fermented cheese whey as a nutraceutical significantly decreased feed consumption and FCR in poultry, while also increasing egg production. While some research, like Sugiharto et al. (2019), found no significant impact of whey powder on broiler FCR, others, such as Samli et al. (2019) and Zarei et al. (2020), observed improved growth performance and FCR when whey was combined with probiotics. Kanza et al. (2019) noted that whey protein concentrate (WPC) supplementation boosted feed consumption, weight gain, and body weight, though without significantly affecting FCR. Ashour et al. (2020) demonstrated improved FCR in all WPC-fed broiler groups compared to controls, with optimal results at 0.20% and 0.25% WPC.
 While FWP generally promote satiety, enhance growth performance, and support overall health and productivity, a crucial point of our study was to directly evaluate fermented whey peptides (FWPs) as a viable alternative to antibiotics in poultry. We found that FWPs, administered for a mere one week (1 mL per day), yielded comparable effects on growth performance to a conventional probiotic and immunomodulator regimen maintained for 42 days. This striking efficiency underscores not only the health benefits of FWP for broiler birds, but also significant potential economic advantages for poultry farmers due to reduced administration duration. This aligns with the broader consumer demand for antibiotic-free poultry, which offers a reduced risk of antimicrobial-resistant (AMR) pathogens and improved gut health in the final product. Future research will further explore FWP’s impact on body fat composition, meat and egg quality, egg production capacity, and meat protein content in broilers.
While FWP generally promote satiety, enhance growth performance, and support overall health and productivity, a crucial point of our study was to directly evaluate fermented whey peptides (FWPs) as a viable alternative to antibiotics in poultry. We found that FWPs, administered for a mere one week (1 mL per day), yielded comparable effects on growth performance to a conventional probiotic and immunomodulator regimen maintained for 42 days. This striking efficiency underscores not only the health benefits of FWP for broiler birds, but also significant potential economic advantages for poultry farmers due to reduced administration duration. This aligns with the broader consumer demand for antibiotic-free poultry, which offers a reduced risk of antimicrobial-resistant (AMR) pathogens and improved gut health in the final product. Future research will further explore FWP’s impact on body fat composition, meat and egg quality, egg production capacity, and meat protein content in broilers.
Supplementation with fermented cheese whey fractions (FWP) significantly reduced serum triglyceride, cholesterol, and total lipid levels in broiler chickens, while no significant effect was observed on high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL) levels over the entire study period as shown in Fig. 4.
Specifically, the lowest serum cholesterol was found in the T3 group (fed <10 kDa Permeate) at 123.75±9.746 mg/dL, followed by T4 (>10 kDa Retentate) at 125.89±8.855 mg/dL, and T2 (<3 kDa Permeate) at 143.21±10.451 mg/dL, all significantly lower than the control group (T1) at 165.09±9.327 mg/dL. Triglyceride levels were also lower in FWP-fed groups: T3 (65.33±5.833 mg/dL), T4 (71.60±3.956 mg/dL), and T2 (72.47±5.778 mg/dL), compared to T1 (85.71±4.128 mg/dL). Total lipids were significantly higher in T1 (625.83±22.97 mg/dL) compared to T2 (559.80±28.562 mg/dL), T4 (528.55±29.697 mg/dL), and T3 (514.73±39.140 mg/dL).
While HDL, a beneficial cholesterol, remained largely similar across all groups (T1: 57.48±7.016 mg/dL; T2: 54.40±6.290 mg/dL; T4: 51.75±6.316 mg/dL; T3: 49.50±8.138 mg/dL), “bad” LDL cholesterol and VLDL cholesterol levels were highest in the control group (T1: LDL 92.81±14.174 mg/dL, VLDL 17.90±1.089 mg/dL) compared to the FWP-fed groups (T2: LDL 74.89±15.667 mg/dL, VLDL 14.46±0.975 mg/dL; T3: LDL 62.14±10.569 mg/dL, VLDL 13.07±2.375 mg/dL; T4: LDL 62.41±16.181 mg/dL, VLDL 14.32±1.757 mg/dL). Despite numerical differences, no statistically significant variations were observed for LDL and VLDL cholesterol across all groups.
The observed reduction in cholesterol, triglyceride, and total lipids in FWP-fed groups is likely attributed to the strong binding capacity of whey peptides with bile acids, which decreases intestinal lipid absorption and promotes cholesterol excretion and de novo bile acid synthesis by the liver. This effect is enhanced in hydrolyzed whey peptides due to changes in sulfhydryl groups and disulfide bonds that facilitate hydrogen bond interaction with bile salts. These findings are consistent with previous research demonstrating that dairy proteins, particularly whey, contribute to satiety and improved lipid profiles.
 Beyond lipid profiles, this study also investigated the effects of FWP on broiler blood parameters and gut metagenomics. While red blood cell (RBC) counts remained within the normal physiological range and showed no significant difference, the FWP-fed groups numerically exhibited higher RBC counts compared to the control (T1: 2.20±0.107×106 /uL; T4: 2.39±0.083×106 /uL). Crucially, a significant increase (p < 0.05) in white blood cell (WBC) counts was observed in FWP-fed groups (e.g., T2: 22.65±0.240×103 /uL) compared to the control (T1: 22.26±0.336×103 /uL), indicating an enhanced immune response, likely due to the immunoregulatory properties of whey peptides. Although mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) showed no significant changes, the numerically higher values in FWP groups suggest an improved capacity to resist stress. Additionally, significantly higher platelet counts were observed in FWP-fed groups (e.g., T4: 126.25±0.854 /µl) compared to the control (T1: 120.00±1.291 /µl), potentially linked to increased levels of vitamins (like B12) and folic acid enhanced by fermentation. Furthermore, FWP supplementation significantly lowered blood glucose levels in broiler chickens, with T4 exhibiting the lowest at 182.82±1.57 mg/dL compared to T1 at 204.27±3.34 mg/dL, likely due to the insulinogenic effects of branched-chain amino acids (BCAAs) abundant in whey protein, or its influence on incretin hormones. These findings suggest FWP’s potential in glycemic control and immune system support, especially for individuals with compromised immune function or glucose metabolism issues.
Beyond lipid profiles, this study also investigated the effects of FWP on broiler blood parameters and gut metagenomics. While red blood cell (RBC) counts remained within the normal physiological range and showed no significant difference, the FWP-fed groups numerically exhibited higher RBC counts compared to the control (T1: 2.20±0.107×106 /uL; T4: 2.39±0.083×106 /uL). Crucially, a significant increase (p < 0.05) in white blood cell (WBC) counts was observed in FWP-fed groups (e.g., T2: 22.65±0.240×103 /uL) compared to the control (T1: 22.26±0.336×103 /uL), indicating an enhanced immune response, likely due to the immunoregulatory properties of whey peptides. Although mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) showed no significant changes, the numerically higher values in FWP groups suggest an improved capacity to resist stress. Additionally, significantly higher platelet counts were observed in FWP-fed groups (e.g., T4: 126.25±0.854 /µl) compared to the control (T1: 120.00±1.291 /µl), potentially linked to increased levels of vitamins (like B12) and folic acid enhanced by fermentation. Furthermore, FWP supplementation significantly lowered blood glucose levels in broiler chickens, with T4 exhibiting the lowest at 182.82±1.57 mg/dL compared to T1 at 204.27±3.34 mg/dL, likely due to the insulinogenic effects of branched-chain amino acids (BCAAs) abundant in whey protein, or its influence on incretin hormones. These findings suggest FWP’s potential in glycemic control and immune system support, especially for individuals with compromised immune function or glucose metabolism issues.
Histological analysis of the heart, intestine, and liver revealed no significant organ toxicity or abnormal morphology in FWP-supplemented groups, confirming the safety of FWP as a dietary additive. This is consistent with the role of whey protein hydrolysates and BCAAs in maintaining tissue health. A metagenomic analysis of cecal samples, encompassing 1.2 million paired reads across 8 samples, identified 1065 unique Amplicon Sequence Variants (ASVs). While alpha and beta diversity indices showed no statistically significant shifts in overall microbial populations between groups, FWP notably modulated gut microbiota composition at the phylum and genus levels, despite the short 7-day administration period. For instance, Bacteroidota, Campylobacterota, Desulfobacterota_I, and Proteobacteria were higher in T3 (<10 kDa permeate), whereas Firmicutes were more abundant in T4 (>10 kDa retentate). Importantly, beneficial bacterial communities were effectively supported, and potentially harmful bacterial loads, such as Alphaproteobacteria, Campylobacteria, Clostridia, and Helicobacter, were observed to be lower in some FWP-treated groups, notably T4, as compared to control. This suggests FWP’s antimicrobial properties and its ability to modulate the gut microbiome, making it a valuable intervention for gut health. Overall, despite a shorter administration period, FWPs demonstrated comparable beneficial effects on gut microbiota to the 42-day commercial probiotic and immunomodulator regimen.
Conclusion
This study concludes that supplementing broiler diets with fermented cheese whey peptide (FWP) fractions synergistically improves growth performance and overall health. FWP supplementation significantly reduced serum cholesterol, triglyceride, and blood glucose levels. Furthermore, FWP exhibited no adverse effects on broiler organs (heart and liver) and, notably, influenced white blood cell (WBC) counts, likely due to their immune-regulating and anti-inflammatory properties. The study highlights FWP’s positive impact on cholesterol activity and its role beyond mere nutrition, offering beneficial physiological effects during broiler growth.
While promising for large-scale antibiotic-free broiler production and potential human functional food or medicinal applications, limitations include the need for standardizing FWP processing parameters for large-scale production and addressing the stability of the liquid FWP product, which requires refrigeration and has a shelf life of up to three weeks. Future research should focus on these aspects, as well as evaluating FWP’s effect on egg production and conducting further clinical research in humans.
Disclaimer: The article is reproduced from original article titled “Influence of fermented whey protein fractions on the growth performance, haematological traits, serum biochemistry, faecal and caeca microbiota of broiler chickens” in Nature Briefing: Translational Research.
by By Bhagyashree Das1, Mansi Desai2, Nikesh J. Bhagora3, Prakash Koringa4, Mohsin Pathan5, J. C. Thakor6,
Fulabhai P. Savaliya7, Shaikh Adil8, and Subrota Hati9
¹ & ⁸: Parul University, Vadodara
²: CHARUSAT Campus
³, ⁴, ⁵, ⁶, ⁷ & ⁹: Kamdhenu University
To read complete article, please refer to the following link https://go.nature.com/4m0oDKL
or scan here










