 Etiology
Etiology
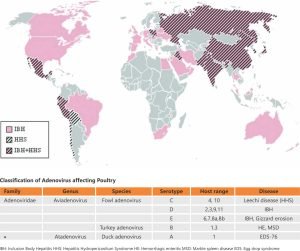
The disease is caused by a virus belonging to the fowl adenovirus (FAdV), which is a member of the family Adenoviridae. FAdV is a non-enveloped, double-stranded DNA virus with a genome size of 43-45 kb. FAdVs are grouped into five species (A-E) based on their molecular structure, specifically their restriction site patterns. Based on cross-neutralization tests, they are further divided into 12 serotypes (FAdV-1 to 8a and 8b to 11).
Epidemiological findings indicate that serotypes 2, 8a, 8b, and 11 cause Infectious Bursal Disease (IBH).
Fowl adenovirus (FAdV) serotype 4 is highly pathogenic for chickens, especially for broilers aged 3 to 5 weeks, and has emerged as one of the foremost causes of economic losses in the poultry industry over the last 30 years. FAdV serotype 4 is the causative agent of hydropericardium syndrome (HPS), a severe disease in broiler chickens characterized by the accumulation of a clear, straw-colored fluid in the pericardial sac, as well as nephritis and hepatitis.
Adenoviruses are resistant to lipid solvents such as ether and chloroform, sodium deoxycholate, trypsin, 2% phenol, and 50% alcohol. They are inactivated by a 1:1,000 concentration of formaldehyde. FAdV is distributed widely throughout the world, and domestic avian species of all ages are susceptible.
deoxycholate, trypsin, 2% phenol, and 50% alcohol. They are inactivated by a 1:1,000 concentration of formaldehyde. FAdV is distributed widely throughout the world, and domestic avian species of all ages are susceptible.
Epidemiology
The disease was first described in the USA in 1963. The first incidence of FAdV-4 in Asia was recorded in Pakistan in 1987. Afterward, the virus spread to extensive regions of Asia, Central and South America, and some European countries. In India, the first outbreak of FAdV-4 was documented in the poultry farms of Jammu and Kashmir, Punjab, and Delhi in 1994. Months later, hydropericardium syndrome (HPS) caused by FAdV-4 spread to other parts of India, including the Terai region of Uttarakhand, Uttar Pradesh, Maharashtra, Andhra Pradesh, Karnataka, Tamil Nadu, and Kerala. The disease is commonly called “leechi disease.”
Transmission
Transmission of FAdVs occurs both vertically (from infected breeders to progeny) and horizontally (through direct contact or contaminated feed/water). Infections with FAdVs are widespread and endemic worldwide. In many cases, the virus is present without any signs of clinical disease. Many layer and breeder flocks in the field test positive for FAdV antibodies. Birds of all age groups are susceptible to FAdV. The severity of lesions depends on the age of the infected birds and the levels of maternal antibodies.
 Clinical Signs and Lesions
Clinical Signs and Lesions
IBH has been documented in chickens as young as 7 to 10 days old, although it typically affects poultry between the ages of 3 to 5 weeks. IBH can impact breeders, broilers, and layer hens. Flocks affected by IBH often experience an abrupt onset of mortality, and individual chickens may exhibit nonspecific clinical signs such as lethargy, huddling, ruffled feathers, and yellow, mucoid droppings due to excess bile acids. IBH is characterized by a sudden onset of mortality that peaks after 3 to 4 days and usually resolves by day 5, though it can occasionally continue for 2 to 3 weeks.
Pathology: Gross and microscopic lesions
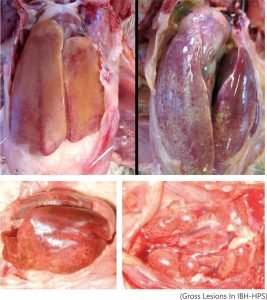
The major liver abnormalities in IBH include pale, friable, enlarged livers with yellowish discoloration and multiple pale hemorrhagic foci. Lymphocytic infiltration, cellular necrosis, and degeneration of infected organs have also been observed within three to nine days post-infection. Additionally, atrophy of the bursa and swelling of the kidneys have been reported in some cases. Broilers are more vulnerable to higher mortality due to severe metabolic imbalances and substantial damage to both the pancreas and liver. Microscopic lesions characteristic of FAdV infection include intranuclear inclusion bodies in the liver.
Metabolic effects in birds
IBH-HPS generally affects bird metabolism. During the peak of infection, broilers may suffer from hypoglycemia, which is associated with severe lesions in the liver and pancreas. It can be concluded that FAdV infections in broiler chickens can lead to metabolic disorders, primarily affecting glucose metabolism in the affected flock.
Diagnosis of Inclusion Body Hepatitis
A tentative diagnosis is usually based on a pattern of spiking mortality and typical gross lesions observed during post-mortem examination. The diagnosis is confirmed through histopathology, microscopic examination of affected tissues, and detection of typical lesions, particularly intranuclear inclusion bodies. Molecular diagnosis can be performed using PCR and real-time PCR with specific primer sequences. Primers are primarily designed based on the hexon gene, and sequencing of its variable regions is used to classify viruses into species.
 Intervention strategies to control IBH
Intervention strategies to control IBH
Management procedures to prevent or minimize IBH disease
Preventing aviadenovirus infection primarily relies on strict biosecurity practices. Effective management practices, such as cleaning and disinfecting farm premises and equipment, restricting entry, and ensuring the use of personal protective equipment for visitors and vaccination crews, play a crucial role in preventing IBH. The resistance of aviadenoviruses to heat inactivation (up to 70°C) and common disinfectants (particularly lipid solvents such as ether, chloroform, and phenol) presents a significant challenge, especially in poultry houses with impervious floors and walls.
Therefore, effective FAdV control begins at the primary breeder level with optimal disinfection and vaccination as complementary strategies to prevent infection and, consequently, protect against vertical transmission. However, horizontal spread is a significant issue that should not be overlooked, and maintaining a commercial flock free of FAdV infection requires considerable effort.
Additionally, affected flocks may exhibit metabolic disorders due to damage to the liver and pancreas, leading to hypoglycemic conditions. Corrective actions include restoring liver and pancreatic function to normal levels, which in turn improves glucose metabolism and reduces mortality in IBH-affected flocks.
Vaccination
In recent years, outbreaks of IBH have led to higher bird mortality compared to earlier, milder outbreaks, resulting in severe economic losses for the poultry sector. Effective vaccination is therefore required to control the infection. Both live and inactivated vaccines are available to combat IBH. Breeders should be administered inactivated vaccines at 12, 18, and 45 weeks, while commercial chicks should be vaccinated on the 0th day during the vulnerable period (June to October). FAdV serotypes 4 and 8 are most commonly used in commercial vaccine preparations. However, for disease prevention and control in endemic areas, it is recommended to use autogenous inactivated vaccines prepared from the prevalent serotype of FAdV. Breeders primarily use strict biosecurity practices and autogenous inactivated vaccines to prevent vertical transmission and ensure the transfer of maternal immunity from breeding flocks to their progeny.
by Dr Ashok Reddy & Dr Mounika, Immeureka Animal Health









